I was reading something yesterday about President Trump’s desire to speed up FDA approvals for new drugs, so I decided to check: how long does FDA approval take these days? Here are the numbers over the past decade:
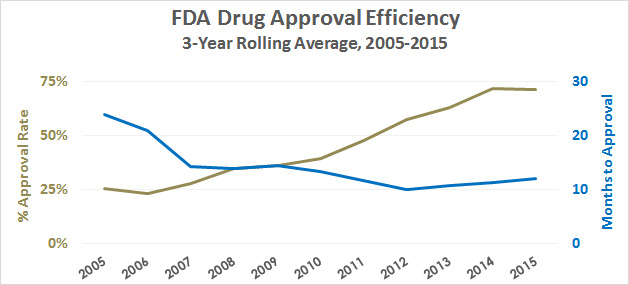
I’ve used a 3-year rolling average to smooth out the spikes, but the trend is pretty obvious. In the past ten years, the time to approve new drugs has been cut in half and the approval rate has tripled. Note that this is only for “standard” drugs, not “priority” drugs, so it’s not contaminated by special treatment given for certain lifesaving compounds.
I’m sympathetic to arguments that our narrow escape from the thalidomide disaster of the early 60s traumatized FDA scientists, and they overreacted by making approvals too hard. The problem is that the lesson of thalidomide approval in Europe isn’t that approvals were done too quickly, it’s that approvals shouldn’t be based on handwaving from pharmaceutical companies. As long as the testing regimen is rigorous enough, there’s no reason that approvals shouldn’t be done in a timely way.
That said, how much faster does Trump want approvals to go? A recent study suggests that the average FDA approval time is now considerably faster than Europe’s, and that “the vast majority” of new drugs were first approved for use in the United States:
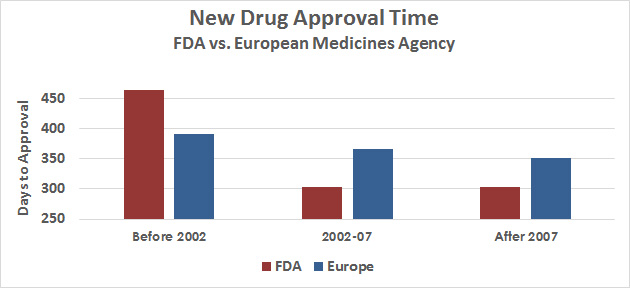
If anything, the FDA may have become too aggressive. They’ve made some far-reaching reforms in only a decade. Ten years from now, the chart to look at will be a comparison of drug catastrophes before and after this change.1
1I don’t mean this in a snarky way. There’s no cosmic “right answer” for how fast new drugs should be approved. It’s all a matter of how much risk we’re willing to take vs. how long we’re willing to delay potentially effective therapies. A decade from now, we’ll need to look back and see just how much extra risk, if any, the FDA has introduced into the system.