John Nacion/SOPA Images via ZUMA
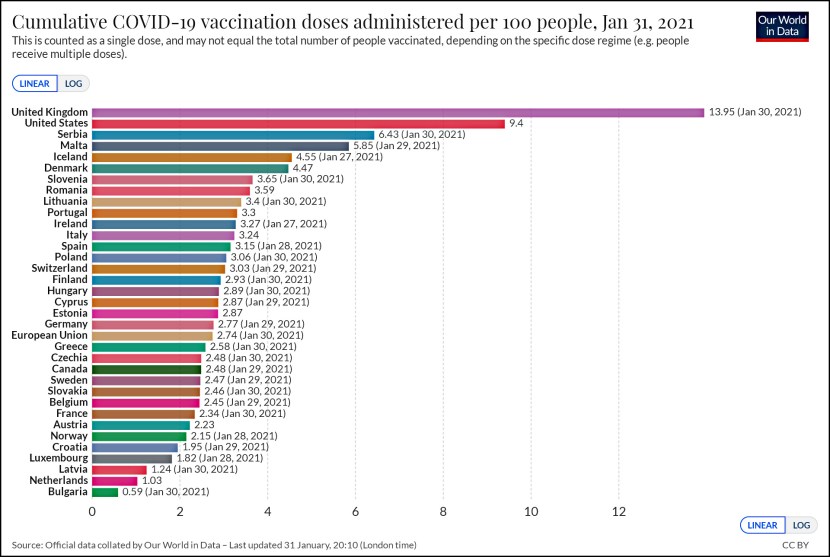
The news of the day is that the federal government contracted for only 100 million doses of the Pfizer vaccine (enough for 50 million people) and declined Pfizer’s offer during the summer to buy more. Now Pfizer says their excess supply has already been promised to other countries, and we won’t be able to get more until June.
This is all true, but keep in mind that the feds also contracted for a bunch of other vaccines. All of these are currently in Phase 3 testing:
- Moderna: 100 million doses (50 million people)
- Johnson & Johnson: 100 million doses (100 million people)
- Sanofi/GlaxoSmithKline: 100 million doses (50 million people)
- AstraZeneca: 300 million doses (150 million people)
- Novavax: 100 million doses (50 million people)
All of these vaccines are behind Pfizer in the race for approval, but manufacturing is ongoing. This means that when they get approved—and at least some of them are bound to be approved over the next month or so—there will be additional doses of vaccine available. Given this timeline, along with the pace at which the vaccines can be rolled out to the public, it’s likely that supply won’t turn out to be a big bottleneck. By the time we run out of Pfizer’s vaccine, others will be online and deliverable.
There’s a lot of speculation here, of course. It’s always possible that no other vaccine will ever be approved and we’ll face a shortage. But it’s really not very likely.