A nurse at a drive-up coronavirus testing station.Ted S. Warren/AP
This story was originally published by ProPublica, a Pulitzer Prize-winning investigative newsroom. Sign up for The Big Story newsletter to receive stories like this one in your inbox.
Nurses at one hospital in southeastern Washington state have alleged that, amid the COVID-19 pandemic, they were ordered by supervisors to use one protective mask per shift, potentially exposing themselves to the novel coronavirus.
At another hospital, just east of Seattle, nurses had to use face shields indefinitely.
At a third hospital, on Washington’s border with Oregon, nurses reported that respirators were expired. The hospital responded, the nurses said, by ordering staff to remove stickers showing that the respirators might be as much as three years out of date.
The accounts these nurses provided are drawn from nine complaints filed by the Washington State Nurses Association with the state Department of Labor & Industries since March 11. They paint a picture of how the first state hit by COVID-19 continues to struggle to provide adequate safety measures for medical workers.
Their struggle may well preview what medical providers in other states could face amid a national shortage of personal protective equipment, or PPE. The complaints from Washington also show the increasing sense of fear, frustration and powerlessness many nurses and other medical workers feel as COVID-19 pummels the health care system.
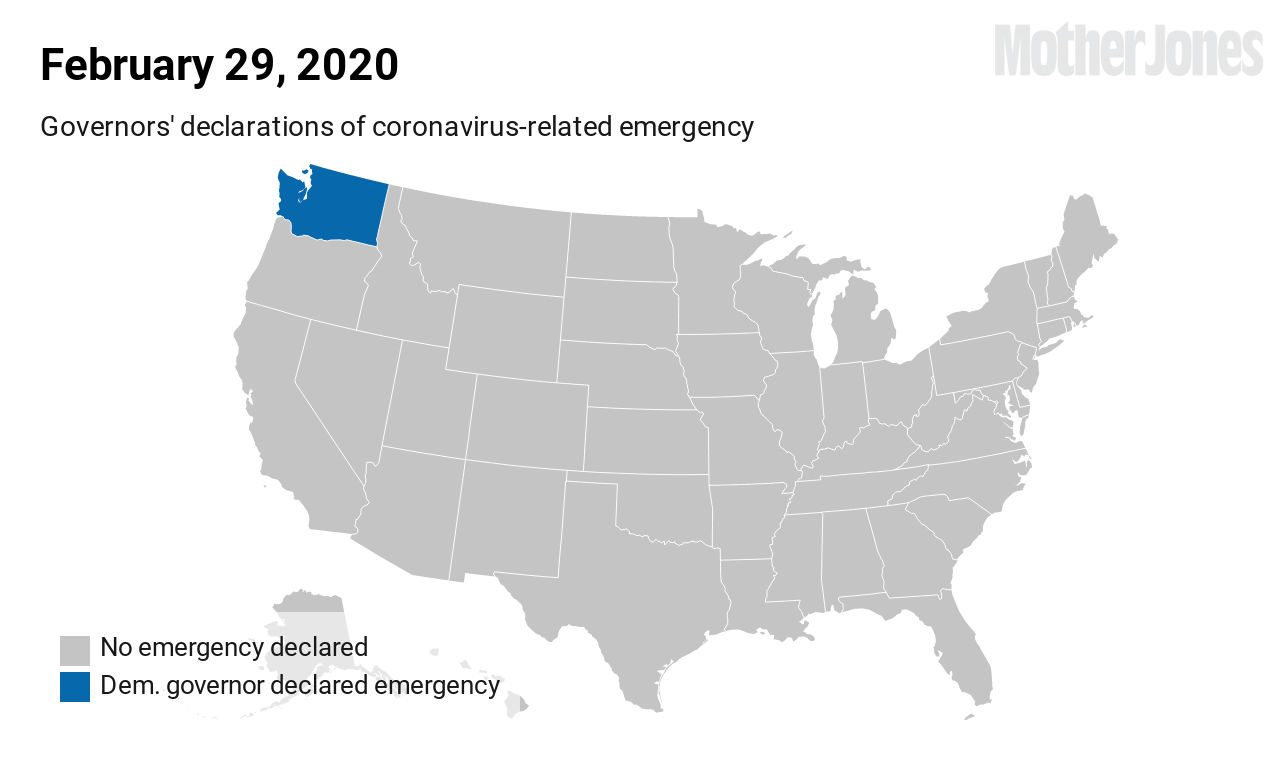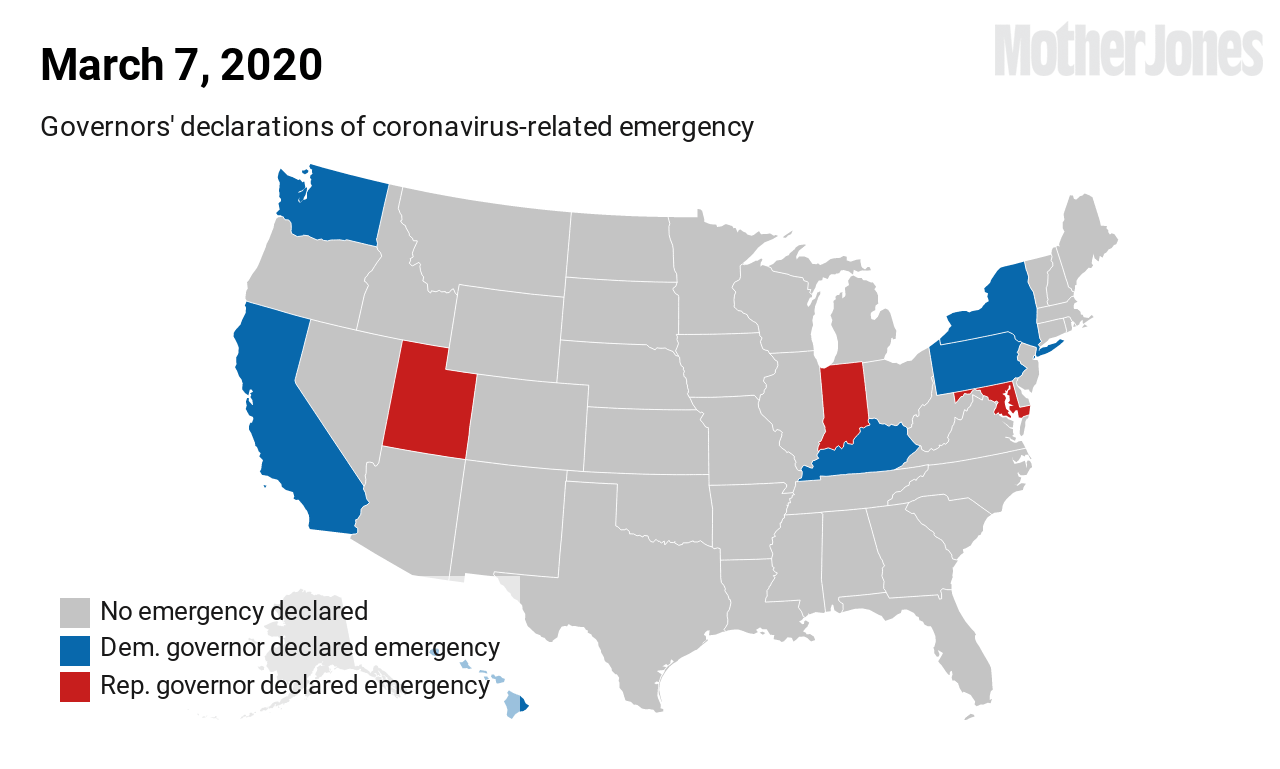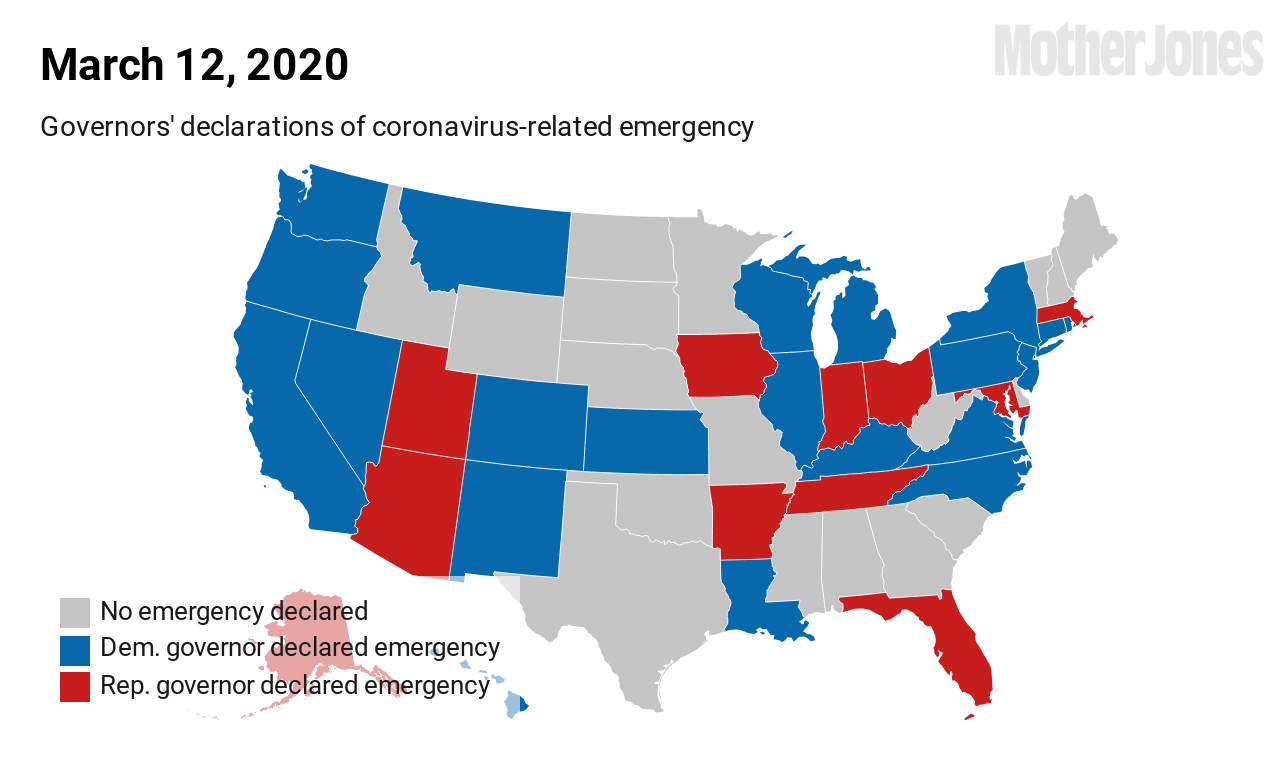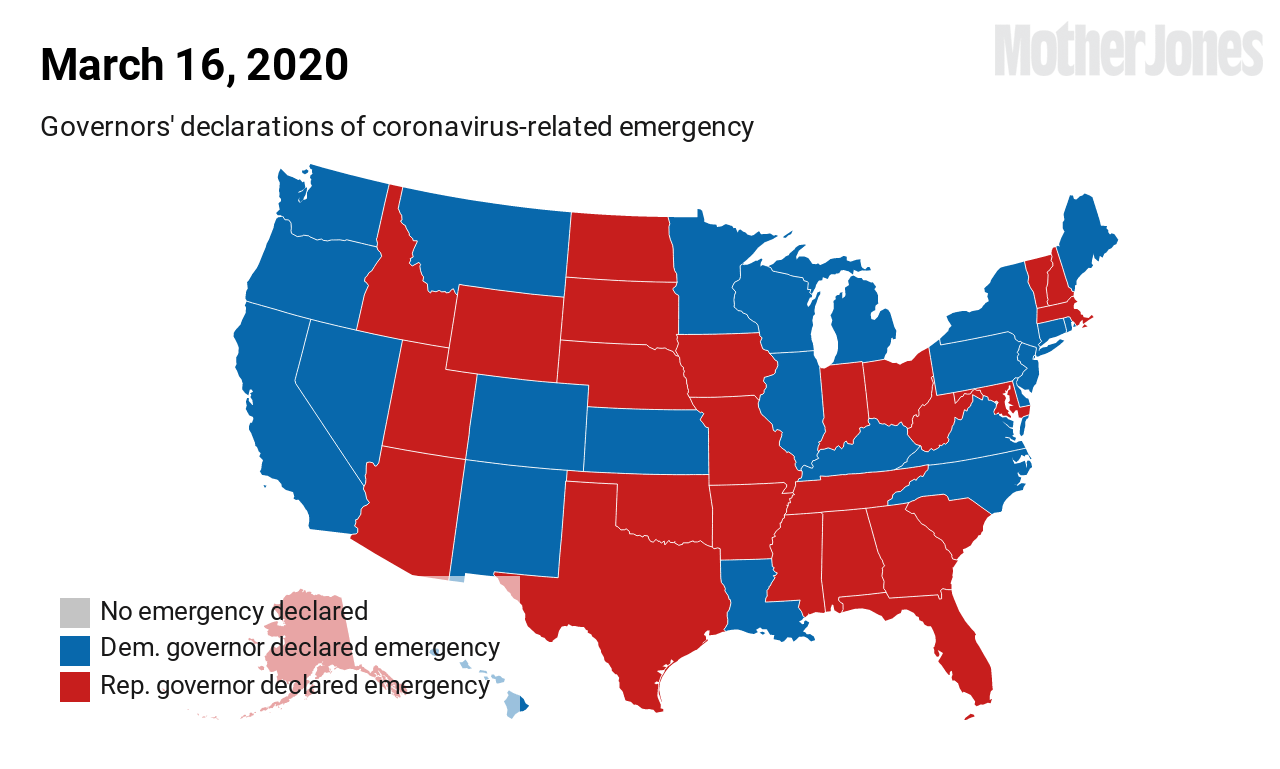
As of this weekend, the Washington Department of Health has reported 3,700 known COVID-19 cases in the state and 175 deaths.
ProPublica contacted all nine hospitals that were the subject of a nursing association complaint. Four responded. They said they were taking measures to protect their employees, but emphasized the unprecedented crisis in which their hospital staffs are now working. In a press briefing Thursday, Washington Gov. Jay Inslee said the federal government had supplied the state with “significant shipments of personal protective equipment” but added that he had “profound long-term concerns about being able to procure these necessities.” Inslee, a vocal critic of the Trump administration, reportedly clashed with the president in a conference call with governors Thursday, according to the Washington Post, pleading with him to take more action.
Some nurses in Washington state told ProPublica that they feel caught between their responsibility to care for patients and their own safety. They believe they have no choice but to keep working, at great personal risk and with limited means to raise concerns within their chains of command. They could be disciplined for talking to the media, and some said they had been explicitly warned about that in emails sent by hospital administrators. To refuse an assignment on safety grounds, they said, could find them ostracized by colleagues or, worse, fired for insubordination.
“It’s a health care war zone,” said a critical care nurse who works at one of the nine hospitals named in the complaints and, like all nurses interviewed for this story, asked to remain anonymous.
She told ProPublica that she has had to reuse masks and other PPE, if she can obtain it at all. She uses a simple surgical mask—a paper cover with ear loops, no eye cover—even when working with patients waiting for COVID-19 tests, because that’s all that’s available. Community members have been asked to donate handmade masks. She wears one over her surgical mask; it doesn’t protect from viruses but at least is one more layer. Every night when she comes home, she strips down in the garage and throws her dirty hospital scrubs in the washer before rushing in to take a shower.
“Never in a million years did we think when we were in nursing school that our employer would not provide us with the PPE they are legally obligated to provide us with, to care for those patients,” she said.
Her supervisors acknowledge the shortage, she said, but have told staff members that unless they make do, they could run out of all protective gear, making their situation even more precarious.
“We take an oath of ‘do no harm,’” the nurse said. “Would we be willing to take care of these patients with nothing?” She has a family, some of whom would be especially susceptible to the disease.
“I don’t know what I would do,” she said. “We are continuing to reuse this equipment so hopefully we don’t have to make that choice.”
“This Is What You Signed Up For”
Nurses in Washington, where the virus first surfaced in the U.S., believe their early experience can help prepare health care workers elsewhere. The Washington State Nurses Association has even produced a list of recommendations for other states, called “Lessons learned from the front lines.” Those lessons include, “Know your employer’s plan for PPE (personal protective equipment),” and “Know the testing and treatment protocols now.”
When the novel coronavirus spread across Washington in February and March, the lack of supplies in hospitals, coupled with uncertainty over what protective measures were needed, presented many nurses with a difficult choice. Nurses given a dangerous job could accept the assignment and its attendant risks, or refuse and face possible discipline. The WSNA, a union that represents more than 17,000 nurses, advised members who refused an assignment to stay and do other jobs. For those nurses who accepted an “abnormally dangerous” assignment, the union advised filling out what is called an ADO form. ADO stands for Assignment Despite Objection.
When ProPublica mentioned ADO forms to some nurses in Washington, they did not react with enthusiasm. “It’s the stuff of fairytales,” said one nurse in the Seattle area who specializes in mental health. “Nurses, administratively, are strongly discouraged to use the forms or outright shamed for documenting what they are uncomfortable with in a caregiving situation.”
Under collective bargaining agreements, nurses disciplined for refusing an assignment can push back, arguing that the discipline lacked “just cause.” But the WSNA has warned its members that given the current national and state emergency declarations, the resolution of any such objection “would likely be delayed and the outcome may be uncertain.”
Ruth Schubert, the association’s communications director, said the WSNA has received about 70 Assignment Despite Objection forms related to the coronavirus. She declined to provide copies, citing confidentiality, but did share excerpts, including one that said: “Continue to be asked to reuse single use masks for COVID-19 modified droplet patients and wear ill-fitting gowns that fall off shoulders. Goggles not available.” Some nurses are unlikely to fill out an ADO for fear “that management will see them as complainers,” Schubert wrote in an email.
The WSNA said nurses fear being disciplined for talking with the media. A doctor from PeaceHealth St. Joseph Medical Center in Bellingham, close to the Canadian border, told the Seattle Times on Friday that he was fired after he raised multiple concerns about the hospital’s lack of protective measures against COVID-19. A spokesperson for PeaceHealth St. Joseph confirmed the doctor was fired but had no comment because the physician was employed by another company, called TeamHealth.
PeaceHealth St. Joseph is one of the nine hospitals the state nursing association has filed a complaint against, for allegedly asking nurses to reuse and share their protective equipment without proper cleaning. As of Saturday morning, PeaceHealth had not responded to ProPublica’s inquiries about this incident or the complaints. TeamHealth, the doctor’s primary employer, told ProPublica that the physician has “not been terminated,” and that TeamHealth is “committed to engaging with him to try to find a path forward. Now more than ever, we need every available doctor, and we will work with [him] to find the right location for him.”
A Seattle nurse who specializes in oncology said her hospital’s administration initially downplayed the risks: “I had a manager come in and tell me, ‘This is just like the common cold.’” The nurse added, “We’re being told, business as usual, this is what you signed up for.”
Some nurses in Washington have turned to Facebook to express their frustrations. (ProPublica isn’t identifying the nurses in these threads, but did confirm their nursing credentials through state licensing records.)
Commenting on a Facebook post that warned against using cloth masks, one registered nurse wrote, “We need to be able to wear something!!!”
A different post linked to a Bloomberg story about hospital workers making masks from supplies bought at craft stores and Home Depot, including industrial tape and foam. “No offense but I’m not wearing someone’s arts and crafts project with this thing,” wrote one registered nurse.
Facebook posts linking to a Tacoma News Tribune story about nurses reusing disposable masks generated multiple me-too threads. “We are doing this,” wrote a nurse in Everett. “Our hospital … also,” wrote a nursing assistant southeast of Seattle. “It’s everywhere,” one RN wrote, followed by a second RN, “This is everywhere,” followed by a third RN, “Yep.”
One nurse told ProPublica that she wrote on Facebook that she had decided to take a break from her job because she could no longer deal with what she considered an unsafe environment.
She was met with criticism by another nurse, who commented that they didn’t get into this field to “cut and run.” That devastated the nurse who spoke to ProPublica, who responded she didn’t “sign up to die.”
The nurse, who works in an eastern Washington hospital, started to get concerned when, on March 10, her hospital loosened some of its PPE guidelines. She is now using up all her vacation and sick leave because she’s nervous to return to work. If she isn’t approved for an extended leave of absence, she said, she is “100 percent prepared to resign.”
“These Are Not Normal Times”
The most recent complaint filed by the state nurses association was on March 23 against Overlake Medical Center. Based in Bellevue, just east of Seattle, Overlake has had dozens of patients with COVID-19 on any given day. On Friday, the number was 40, said Morgan Brice, a hospital spokeswoman. At least 11 patients have died at Overlake from COVID-19, according to Brice. A number of them arrived at the hospital under “comfort care,” meaning their death was imminent and the hospital made efforts to keep them comfortable in their final days.
The complaint filed with the Department of Labor & Industries said nurses were being required to reuse face shields “indefinitely.” “They must clean them themselves and … store in their own locker for reuse day after day, until the chinstrap is loose,” the complaint says, adding: “RNs report the chinstrap is loose after one 12-hr shift.” The complaint also said the hospital was failing to make sure that notification of exposure was reaching nurses on their days off, “thus prompting additional community and family exposure.”
Brice, in an email, told ProPublica that Overlake had yet to receive a copy of the complaint and would not respond to the specific allegations until it has. But the hospital, she wrote, is “committed to investigating the facts related to any complaint and acting appropriately.” She outlined some steps Overlake has taken during the outbreak, including having a team of nurses “committed to the health of our employees.”
“We have dedicated extensive resources to training staff on how to use and maintain their PPE,” Brice wrote. “We have posted videos, daily FAQs for staff, formed a PPE float team to help guide employees, along with our managers rounding the floors on a consistent basis. We know we have taken extraordinary and proper measures to protect the health and safety of our staff, while we respond to the medical challenges being presented on a daily basis.”
The complaints filed with Labor & Industries use a fill-in-the-blank form, with a narrative section to describe alleged hazards.
On March 11, the nurses association filed a complaint against St. Joseph Medical Center in Tacoma, saying nurses were being directed to reuse and share protective equipment. The complaint also alleged that nurses weren‘t being fit-tested for N95 masks, a protective respiratory device worn over the face. Masks that aren‘t properly fitted to a person‘s face can admit contaminated air.
CHI Franciscan, the medical system that includes Tacoma‘s St. Joseph, said it is cooperating with the investigation but was told by the Department of Labor & Industries that no action is required at this time. The system denied that nurses “have been or will be asked to use PPE in a manner not in compliance with CDC, FDA and DOH guidelines,” according to an emailed statement from Cary Evans, the company‘s vice president for communications and government affairs.
The hospital is operating with “7-12 days of PPE” and said it has not had a situation where demand for PPE exceeded supply. Administrators have expanded fit testing for N95 masks. They are also accepting donations of PPE gear from the community. Normally, a 30-day PPE supply is preferred, according to the Washington State Hospital Association.
“These are not normal times,” Evans‘ statement said, “and we are doing everything we can to keep our staff and patients safe, while also conserving masks under the latest local CDC guidelines.”
The same day it filed the St. Joseph complaint, the nurses association submitted seven others, two against hospitals within the same medical system: Multicare Tacoma General Hospital and Multicare Good Samaritan Hospital in Puyallup, a community southeast of Tacoma. A spokesperson for the system wrote that all employees “have the appropriate personal protective equipment (PPE) they need today to do their jobs safely” and noted that hospital staff are allowed “to preserve PPE resources needed to care for our most critical patients.”
“Due to supply chain disruptions, health systems worldwide are dealing with shortages of PPE,” the statement read.
Another complaint, filed against PeaceHealth Southwest Medical Center, a hospital in Vancouver, across the Columbia River from Portland, Oregon, said nurses were reporting lack of access to masks and respirators. When the nurses reported that respirators were outdated, the hospital “directed staff to remove outdated 2017 and 2019 ‘service by‘ stickers on equipment,” the complaint said.
The hospital did not respond to requests for comment from ProPublica as of Saturday morning.
Beth Zborowski, senior vice president of membership engagement and communications for the Washington State Hospital Association, said a lack of PPE is probably the medical community‘s top problem in the state, in terms of its efforts to fight COVID-19. The association advises hospitals to follow Department of Health and CDC recommendations, though many nurses say the latter keeps changing.
“Prior to the pandemic, masks were available on carts outside of rooms,” Zborowski said. “What started happening is those things started disappearing pretty quick. People had to put conservation measures in.” It‘s one reason the state canceled elective procedures in recent weeks.
It‘s unclear how many health care workers in the state may have become ill as a result of COVID-19, though a doctor at EvergreenHealth near Seattle has been infected and Schubert, of the state nursing association, said she knows of nurses who have become sick. Zborowski said the state hospital association does not have a formal record but added she has not heard about many front-line medical workers becoming ill, as they have in New York and Italy. She hopes that means the conservation and safety measures hospitals are taking are working. The goal is to preserve the PPE; otherwise, “I think we will start to see health care workers getting sick.”
Eileen Ravella, a physician assistant at an urgent care facility in Olympia, said her employer is doing well under the circumstances, trying to keep COVID-19 cases cordoned off from other patients and using a drive-through testing area they set up to meet the need. This is helping them preserve PPE, but she knows the system is breaking under the weight of the pandemic.
“I think we all have to step up and do our best despite the obstacles,” Ravella said. “Those patients need us.”
A nurse who works in a western Washington emergency room said that a few weeks into the pandemic, the crisis conditions had begun to feel normal, “which is kind of horrible, too.”
Now she‘s advising nurses in other states about what she‘s experienced. Initially, many of them refused to take her seriously.
She admits that she downplayed COVID-19 at first. Then, in mid-March, she found out about the EvergreenHealth doctor who had contracted the virus.
“It became really real then that some of us may not make it out of here alive,” she said.
A few days later, she and her colleagues received a message from their hospital administrator, advising them to complete their advanced directives—basically a living will.
“[N]ow with COVID-19 making who gets sick an unpredictable event,” the message read, “it‘s an important time to get this done.”